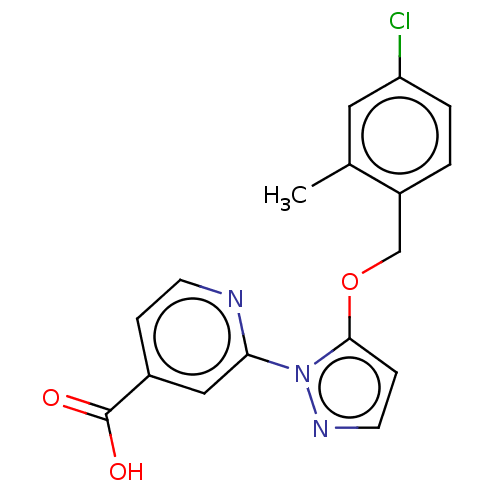
BDBM191600 2-(5-((4-chloro-2-methylbenzyl)oxy)-1Hpyrazol-1-yl)isonicotinic acid (N19)::US10173996, Example 89::US9604961, Example 89::US9714230, 89::US9908865, Example 89
SMILES Cc1cc(Cl)ccc1COc1ccnn1-c1cc(ccn1)C(O)=O
InChI Key InChIKey=PSOBPHXKKHPWMU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 191600
Found 7 hits for monomerid = 191600
Affinity DataIC50: 175nMpH: 7.2 T: 2°CAssay Description:Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Inhibition of KDM5B in human ZR-75-1 cells assessed as reduction in H3K4me3 demethylation incubated for 72 hrs by fluorometric immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Inhibition of recombinant human N-terminal His-FLAG-tagged KDM5B (2 to 751 residues) expressed in baculovirus infected sf9 cells using biotin-H3K4me3...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of KDM5B (unknown origin) preincubated with enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of full length human KDM5B expressed in baculovirus expression system using (ARTK(me3)QTARKSTGGKAPRKQLA peptide substMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of N-terminal his6-tagged human KDM5B (1 to 769 residues) expressed in sf9 insect cells incubated for 20 mins by Alphascreen assayMore data for this Ligand-Target Pair